Krystal's commitment to patient safety is clear and we will advocate for it always. That is why it is so important to keep you up to date with the optimal and cost-effective solutions we offer to reduce healthcare associated infections (HAIs.)
Healthcare associated infections are diseases caused by micro-organisms such as bacteria, viruses, fungi, or parasites while receiving care or services in a healthcare facility.
According to the Centers for Disease Control and Prevention (CDC), 1 in 25 hospitalized patients will get an infection as a result of the care they receive, and an estimated 75,000 patients will die each year, costing the healthcare industry $6 billion annually.
Infection preventionists play a key role in developing assertive strategies that focus on preventing this from happening. These strategies include simple things like regular hand hygiene, consistent use of aseptic techniques, and cleaning and disinfection practices. And more complex precautions that go from patient education to the use of proper solutions specifically designed to reduce the risk of HAIs like physical barriers such as probe covers and drapes.
Even though there are guidelines for disinfecting and using probes, they are not always being put into practice by all stakeholders in the facility. Inadequate infection prevention practices have been associated with ultrasound probe uses that have also been linked to an increased infection risk, outbreaks, and sometimes death.
According to an article published by the American Journal of Infection Control on ScienceDirect with data extracted from a survey of U.S. infection preventionists, surface probes used in invasive procedures were not high-level disinfected or sterilized 15% to 78% of the time. Additionally, 5% to 47% of invasive procedures did not use sterile gel. Of the participants, 20% were aware of instances where an ultrasound probe was used but was not correctly reprocessed.
Ultrasound advances have brought a lot of benefits to the healthcare industry. Still, some professionals underestimate the risk of cross-contamination, exposing patients to infections that can be prevented with a proper safety protocol.
All ultrasound users should follow the appropriate methods to clean and reprocess transducers between exams and in this way, ensure the safety of patients receiving care.

Guidelines for Ultrasound Disinfection
According to the CDC, there are disinfection guidelines for each procedure type to ensure patient safety and reduce the risk of infection. They are based on the Spaulding classification and are divided into three categories: sterilization, high-level disinfection (HLD), and low-level disinfection (LLD.)
1. Sterilization must be mandatory on all critical medical and surgical devices and instruments that enter sterile tissue or the vascular system or through which a sterile body fluid flows (ie blood).
Probes used for these invasive procedures should be sterilized after each use. This process typically includes an autoclave machine (steam sterilizer) that uses steam under pressure to kill harmful bacteria, viruses, fungi, and spore. However, ultrasound devices cannot be autoclaved because they are heat sensitive. For these cases, they can be reprocessed using a high-level disinfection process.
2. High-level disinfection must be provided for semicritical patient-care equipment like endocavity ultrasound devices, gastrointestinal endoscopes, endotracheal tubes, breathing circuits, and respiratory therapy equipment. This guideline must be executed on every device that touches mucous membranes or nonintact skin.
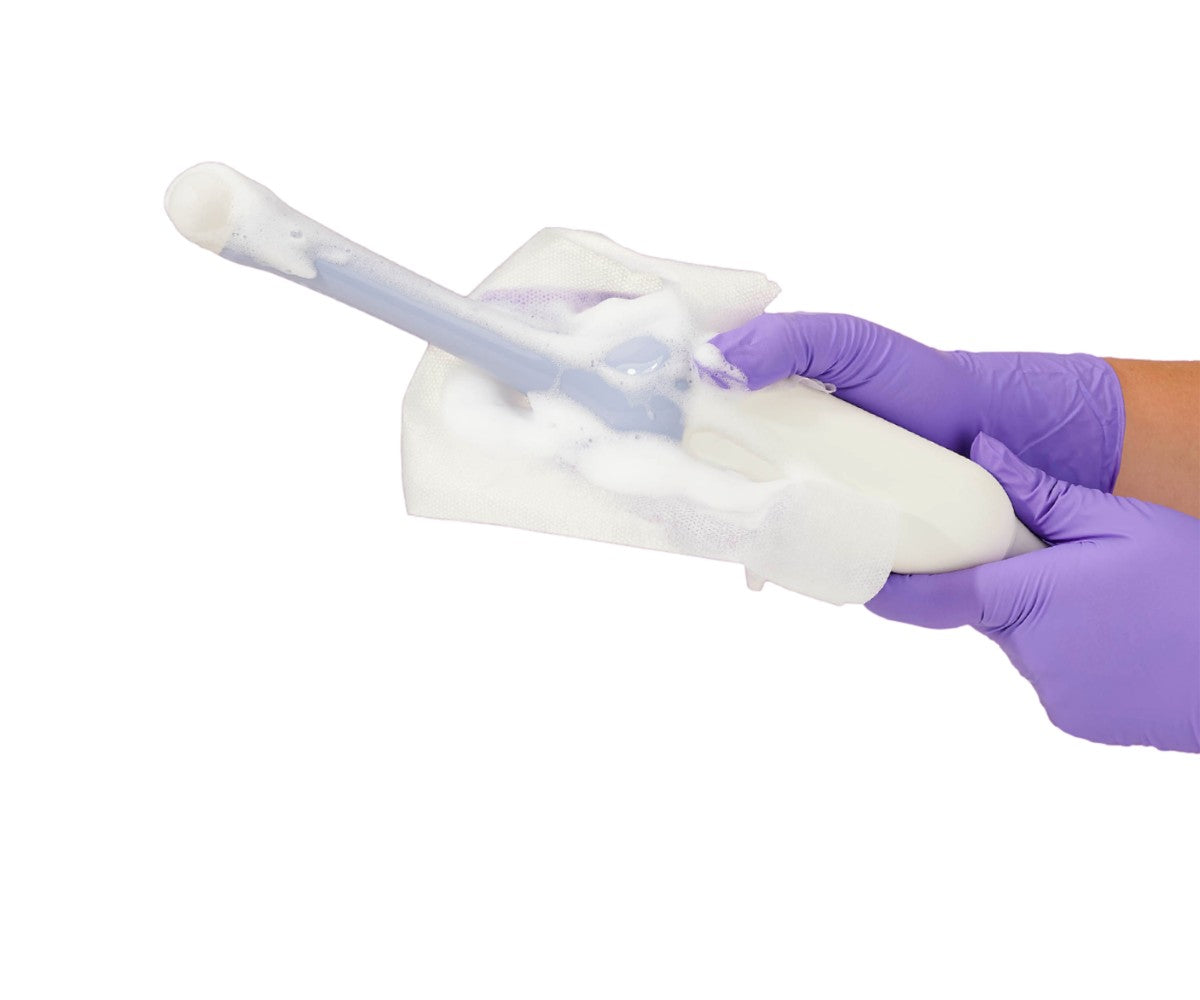
The HLD process can be executed manually using a commercially available germicidal soak or automatically with an automated probe reprocessor. Either way, an initial precleaning step is highly recommended to remove gel and contaminants, especially if the probe is not going to be reprocessed immediately.
It’s important to clean the device as soon as possible after each use when the substances (gel and contaminants) are still wet. Once they dry, it’s harder to clean them up, which can reduce the effectiveness of the high-level disinfection.
When taking the probe to the reprocessing room and before doing the HLD, it must be properly cleaned and dried because most disinfectants act more quickly on clean and dry surfaces, and if there is residual gel or organic matter, they can create a barrier to the disinfectant which reduces its efficacy.
The specific steps to perform high-level disinfection will depend on the products the facility uses. However, the FDA has a list of approved sterilant and HLD products, which have their own manufacturer instructions. Ultrasound users should adhere to those instructions to optimize the disinfection process and ensure patient safety.
3. Low-level disinfection (LLD) should be performed on noncritical patient-care surfaces like bedrails, over-the-bed tables, and equipment such as blood pressure cuffs, and any object that touches intact skin. The general process for ultrasound low-level disinfection consists of removing the transducer connector from the scanner and putting the probe under running water to remove residual gel and organic residue. Then the probe should be cleaned thoroughly with a non-abrasive liquid soap on a damp, soft cloth or gauze pad.
If the probe was used in an interventional procedure, the needle guide should be cleaned to eliminate residual organic matter or gel. After this, rinse the probe again with running water and dry it with a soft cloth or soft paper towels.

To finish, wipe the probe with a probe-compatible low-level disinfectant and make sure that the device is dry before storing or using it again.
Reducing the Risk of Healthcare-Associated Infections with Krystal
Probe covers are an essential part of infection prevention because their use reduces the risk of cross-contamination between the patient and the transducer, thereby making the disinfection process faster and more efficient. This does not mean that using a probe cover allows you to skip the adequate decontamination procedure.
After providing examinations to patients with a single-use probe cover, the ultrasound professional must clean and disinfect the probe to avoid and prevent any risk of cross-contamination.
The practitioner is responsible for ensuring that probe covers are made of high-quality and tear-resistant materials such as Krystal’s that guarantee the protection and safety that patients need.
Krystal offers a wide range of single-use covers that are appropriate for general purpose examinations, invasive procedures, and endocavity ultrasounds. Depending on your need, you will find a solution that covers your expectations and provides your patients with a safe and comfortable experience, even for the latex allergic ones.
While some ultrasound users and sonographers believe that their patients are protected from infection risk by using makeshift barrier shields like condoms, research has shown that up to 13% of condoms fail. This is the main reason behind the importance of choosing an adequate product specifically designed for ultrasound examinations like probe covers.
Having your patients in mind, Krystal developed probe covers that have been meticulously engineered to reduce their potential effect on image quality while protecting the patient and equipment from cross-contamination.
Krystal ultrasound probe covers are designed to work as a physical barrier between the patient and the transducer to reduce the risk of HAIs. Available in two latex-free materials (polyurethane and polyethylene) or latex (for endocavity probes), Krystal covers are FDA-approved and CE-marked.

Healthcare professionals across the US and Europe have made Krystal their go-to option when it comes to high-quality standards and cost-efficiency. Improving patient care begins with enhancing infection control standards, and this is what our ultrasound solutions have been designed for.
Our true north has always been listening to, advocating for, and serving ultrasound users. Krystal solutions guarantee the lowest cost possible, Krystal clear results each time, and a comfortable and safe experience for your patients.
Our mission goes high and beyond. We don't want you to just reduce the risk but to also eliminate it wherever possible while keeping your budget optimized. That’s why you will find complementary products in our catalog specially designed to give you a complete experience that covers all your disinfection and prevention needs cost-effectively.
From ultrasound probe covers for general purpose and interventional procedures as well as endocavity exams to cleaning and disinfection in your healthcare facility, EDM has your back and your needs 100% covered all in one place.
*It is important to note that ultrasound transducer covers DO NOT replace proper disinfection. Furthermore, the Centers for Disease Control and Prevention (CDC) require all semi-critical instruments (in contact with mucous membrane) to undergo high-level disinfection after each patient use.