Description
Tristel ULT — High-Level Disinfectant (HLD) Foam for Ultrasound Probes
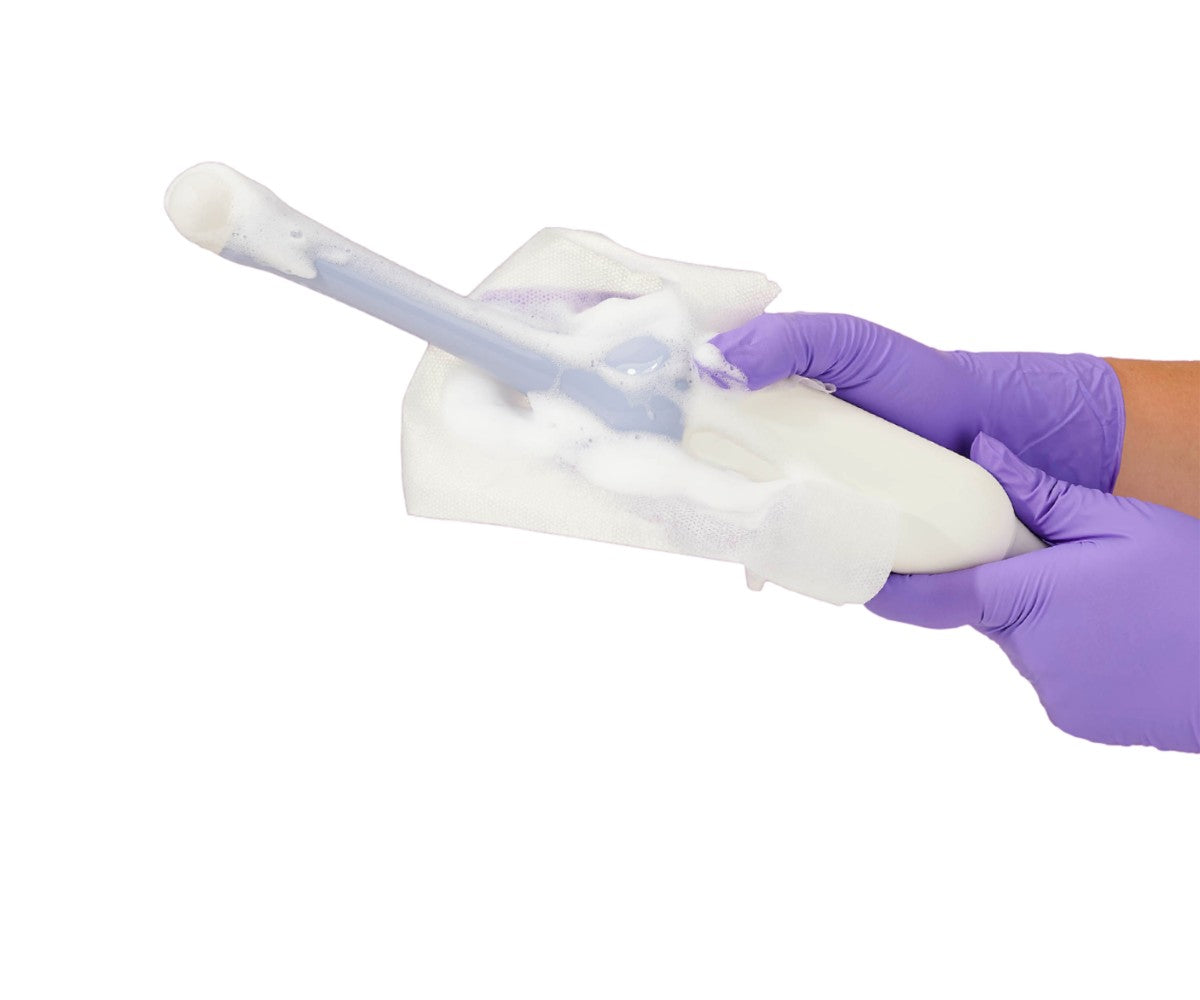
Tristel ULT is a first of its kind: an innovative spray foam that performs high-level disinfection in just 2 minutes. No soaking chemicals or reprocessing equipment necessary: just spray, let sit, and wipe. It’s the easiest and fastest HLD solution for ultrasound probes, now FDA-approved and compatible with over 1,000 ultrasound probes and counting (GE Healthcare, Philips, Samsung, Mindray, and more).
Since 2008, Tristel ULT has been a trusted solution in 35 countries and is highly valued by hospitals, Ob/Gyn, and urology clinics alike, not only for its efficiency and affordability, but also for its effectiveness in the inactivation of HPV types 16 and 18—something not all soaking chemicals are able to accomplish.
At a glance
- Chemistry: chlorine dioxide foam
- Intended use: HLD for ultrasound probes per IFU
- Typical contact time: 2 minutes for HLD (per manufacturer/FDA documentation)
- Set coverage: ~75 HLD cycles per box
- What’s included (per box): 1 dual-compartment bottle (activator/base) + 1 canister of wipes
- Packaging/unit of measure: 1 box = ~75 cycles
Why clinics choose Tristel ULT
- Fast HLD cycle reduces room downtime
- Point-of-care, non-immersive workflow
- Consistent results with simple, repeatable steps
- Compact footprint with no capital equipment
- Supported by microbiological efficacy data (see download section)
What’s in the box
- One dual-compartment bottle of Tristel ULT solution (activated at use)
- One canister of wipes for application and removal
- Covers approximately 75 HLD cycles per box
How it’s used (summary; always follow the IFU)
- Pre-clean the probe
- Activate the bottle and apply foam for the required 2-minute contact time
- Remove residues according to the IFU and complete post-procedure steps
(Refer to the official IFU for full instructions, indications, and limitations — available in the Download section.)
Compatibility
Designed for HLD of ultrasound probes when used per the manufacturer’s instructions. For the latest model-specific status, consult the Probe Compatibility Summary provided in the Download section.
Regulatory & compliance notes
- Prescription device; follows applicable labeling requirements (e.g., 21 CFR 801.109)
- Use according to institutional HLD policies and chemical-handling guidelines
- Refer to the IFU for safety, contraindications, storage, and disposal instructions
Resources (Downloads Available Below)
- Instructions for Use (IFU)
- Safety Data Sheets (Activator, Base, Working Solution)
- Probe Compatibility Summary (1,000+ probes)
- Microbiological Efficacy Summary
- 3T Phone Brochure & Sell Sheet (workflow overview)
- Audit Log Book template for record-keeping
Frequently asked questions
How many cycles does a box provide?
Approximately 75 HLD cycles per box, depending on workflow.
Is the contact time really 2 minutes?
Yes. Tristel ULT is indicated for a 2-minute HLD contact time per the manufacturer/FDA documentation. Refer to the IFU in the Download section.
Can I use it on all my probes?
Check the latest Probe Compatibility Summary in the Download section or refer to Tristel’s live compatibility checker.
What if my infection-prevention team needs documentation?
We provide IFU, SDS, compatibility information, and efficacy summaries directly in the Download section. Additional documentation may be available upon request, pending approval.
For more information regarding Tristel ULT, please contact our sales team at +1 (877) 220-4567 or via live chat on our website.
Payment & Security
Your payment information is processed securely. We do not store credit card details nor have access to your credit card information.