Disinfection in healthcare facilities is a complex part of a proper patient safety strategy. First, cleaning is a key aspect to achieve proper disinfection, the two are inherently tied together. Second, depending on the medical device or surface, and more precisely how the patient interacts with it, different hospital disinfectants or different protocols need to be used.
To help us understand how to properly use disinfectants in hospital settings, we will extract relevant information from the “Guideline for Disinfection and Sterilization in Healthcare Facilities” published by the CDC in 2008 (and updated in 2019). First, we will conduct an overview of disinfection and cleaning in healthcare settings. We’ll proceed by focusing on the reprocessing of endoscopes, followed by the recommendations regarding surface disinfection, and we’ll end this article with effective ways to inactivate Clostridium Difficile. With these best practices in mind, clinicians will be able to properly choose hospital disinfectants.
Disinfection and cleaning
As we already established, proper disinfection involves cleaning. The CDC states that cleaning is one of the factors that impacts the effectiveness of disinfection: it can either nullify or limit it, or bolster it by paving the way prior to the disinfection process.
The goal of cleaning is to remove visible soil from objects and surfaces. It is accomplished manually or mechanically using water with detergent or enzymatic products. Any soil remaining on the surface of instruments interferes with the effectiveness of disinfection, thus making the cleaning process which comes before all the more important.
Disinfectants are classified in three categories. High-level hospital disinfectants will kill all microorganisms except most bacterial spores with a short exposure period. However, with prolonged (3-12 hours) exposure, they can kill spores. Intermediate-level disinfectants will eliminate mycobacteria, vegetative bacteria, most viruses, and most fungi. Low-level disinfectants can kill most vegetative bacteria, some fungi, and some viruses, and they are fully effective in a relatively short period (≤10 minutes).
To know which level of hospital disinfectant should be used for patient-care items and equipment, we use the Spaulding classification, dividing them in three distinct groups:
Critical devices and instruments
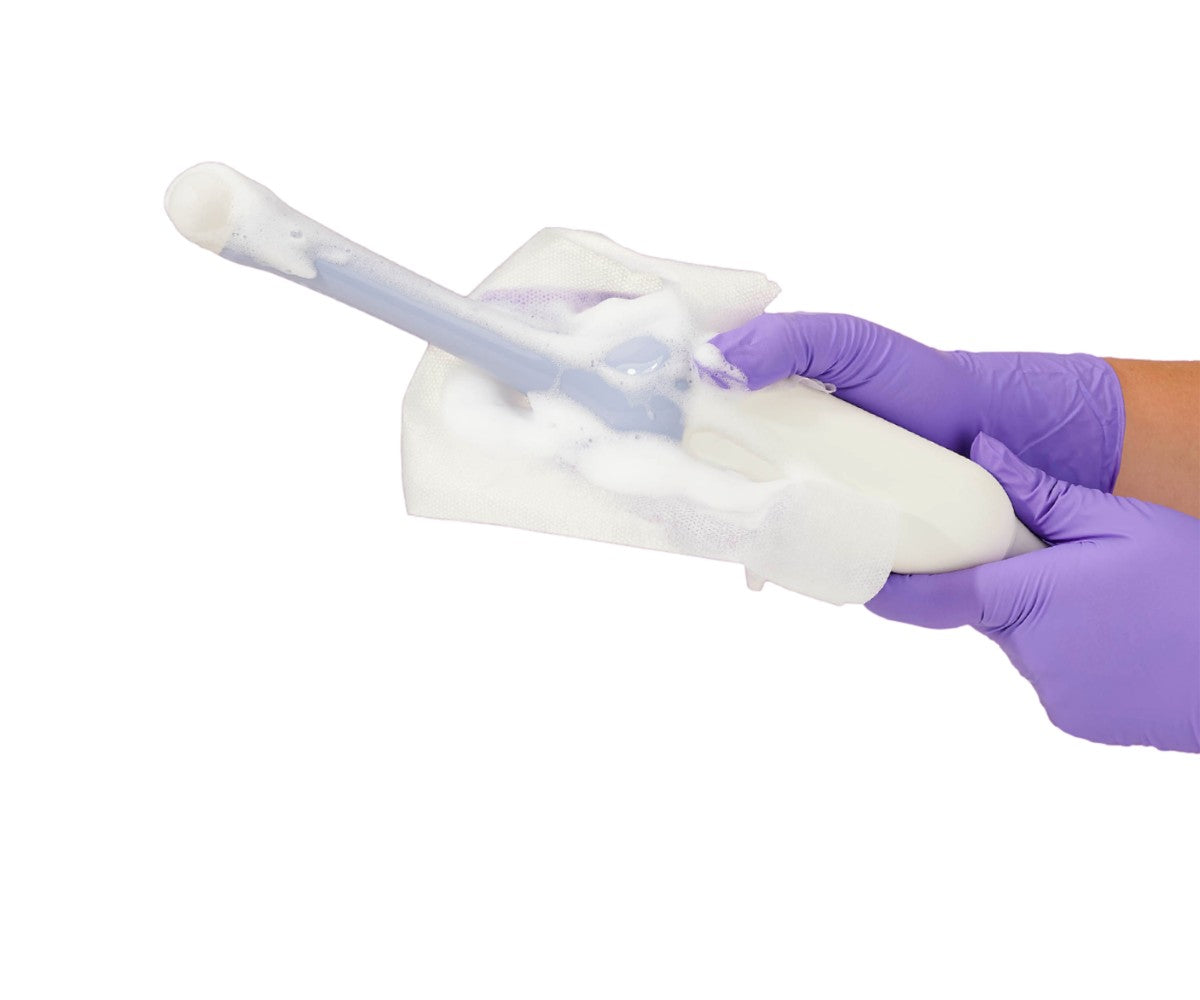
These devices or tools enter sterile tissue or the vascular system, therefore they should be sterile: any microbial contamination could transmit disease. Surgical instruments and ultrasound probes used in sterile cavities are examples of critical items. In some cases where sterilization is not an option, chemical sterilization can be achieved with the help of a high-level hospital disinfectant, provided that guidelines specified by the manufacturer are followed, such as concentration, contact time, temperature, and pH.
Semicritical devices and instruments
Semicritical items come into contact with mucous membranes or nonintact skin. Examples of items in this category are respiratory therapy and anesthesia equipment, some endoscopes and laryngoscope blades. They should be free of all microorganisms, but bacterial spores are permissible. High-level disinfection is required, after thorough cleaning.
Some items in this category that may come into contact with nonintact skin for a brief period of time, such as bed side rails, are usually considered noncritical surfaces and are disinfected with intermediate-level hospital disinfectants.
Noncritical devices and instruments
These medical devices come into contact with intact skin but not with mucous membranes. Since intact skin acts as an effective barrier to most microorganisms, sterility of such items is not critical. Examples of noncritical items are blood pressure cuffs, crutches, computers, bedrails, patient furniture, and floors. They should be disinfected using a low-level hospital disinfectant.
Reprocessing of endoscopes
Endoscopes are a valuable diagnostic and therapeutic tool in modern medicine. Moreover, the incidence of infection associated with their use is very low. Unfortunately, more healthcare-associated outbreaks have been linked to contaminated endoscopes than to any other medical device. The devices must be cleaned and disinfected at least with a high-level hospital disinfectant, or when it’s feasible, they should be sterilized.

The CDC’s main recommendations to achieve high-level disinfection are:
- Detect damaged endoscopes. If they leak, they need to be fixed before used again.
- Meticulously clean the endoscope with an enzymatic cleaner compatible with the endoscope.
- Disconnect and disassemble endoscopic components to completely immerse them in the enzymatic cleaner. If possible, these parts should be steam sterilized.
- Flush and brush all accessible channels to remove all organic residue. Clean the external surfaces and accessories of the devices. This should be done using appropriate cleaning brushes.
- Enzymatic cleaners should be discarded after each use.
- Endoscopes that pass through normally sterile tissues should be processed by using a sterilization procedure before each use. If this is not feasible, high-level hospital disinfection should be provided, followed by a sterile water rinse.
More recommendations can be found in their guidelines, detailing special cases and giving information regarding the different types of disinfectants available.
Surface disinfection with hospital disinfectants
According to the Spaulding classification, surfaces in non-interventional settings are considered non-critical as they usually only come into contact with intact skin. Moreover, the CDC’s guidelines separate environmental surfaces and medical equipment surfaces.
Medical equipment surfaces, such as blood pressure cuffs or X-ray machines, can contribute to the spread of healthcare-associated infections, as they can become contaminated with infectious agents. The infection control guidelines stipulate that these surfaces should be disinfected using a low- or intermediate-level of hospital disinfectant, providing antimicrobial activity.

On the other hand, the reasoning for environmental surfaces is a bit different. First, it’s important to note that for quite some time, disinfecting them was controversial. For example, small studies (of short duration) have shown no difference in healthcare associated infections when floors are cleaned with detergent, and when they are disinfected. Then, a second issue is the contact time required for the hospital disinfectant. Some products require a contact time of 10 minutes; however, some healthcare facilities apply the disinfectant and allow it to dry, spending only around 1 minute. Fortunately, many scientific studies have shown that even 30 to 60 seconds can lead to significant microbial reduction. Also, hospital-grade disinfectants have been introduced with a contact time of one to three minutes, solving this issue. It is important to note that a scientific study demonstrated that when mops are used with detergent, they become infected, and can therefore become a vector for contamination.
The CDC recommends using the same hospital disinfectant for environmental and medical equipment surfaces (if the medical equipment doesn’t require a special treatment due to its fragility), simplifying both training and practice, thereby leading to less errors. Medical equipment surfaces should be disinfected using a low-level hospital disinfectant when visibly soiled and on a regular basis (after each patient or once daily or once weekly). Environmental surfaces should be disinfected using a low-level hospital disinfectant as well, on a regular basis (once a day) and every time spills occur, and when they are visibly soiled.
Many hospital disinfectants suited for surfaces and a wide array of equipment come in convenient, easy to use formats. Disinfectant wipes, such as SONO wipes, eliminate 99.9% of bacteria and viruses in just 15 seconds and are effective against MRSA, COVID-19, Staph, and more. Wipes such as these are even safe to use on sensitive pieces of medical equipment, making them a versatile solution. Users can also select a disinfectant spray, such as Parker Protex, if they prefer this format as it is equally effective against a range of healthcare-associated infections, including HIV, HPV, and coronavirus.
Inactivating Clostridium Difficile with hospital disinfectants
While researchers are not certain about the source of healthcare associated acquisition of Clostridium Difficile in nonepidemic settings, contaminated environments and transmission via the hands of hospital personnel have been considered possible sources of infection. The CDC recommends using products specifically designed to inactivate C. difficile spores in units with high C. difficile rates, combined with appropriate hand hygiene, barrier precautions, and meticulous environmental cleaning. Acidified bleach and regular bleach can inactivate 106 C. difficile spores in ≤10 minutes.
Medical devices, when they are contaminated, can be vehicles for transmission. Examples of such devices include colonoscopes and thermometers. Infection control experts recommend a combination of disinfectants and exposure times, and here are two examples:
- 2% glutaraldehyde and peracetic acid can reliably kill C. difficile spores with an exposure time of 5-10 minutes.
- Ortho-Phthalaldehyde and 0.2% peracetic acid can inactivate enough C. difficile spores in 10-12 minutes at 20°C.
Overall, the use of hospital disinfectants on both surfaces and equipment is pivotal in the fight against healthcare-associated infections, such as C. difficile and MRSA. Through proper training and education, healthcare facilities can strengthen their infection prevention and control programs. Using the right disinfectant, however, does not replace the importance of ensuring that every stakeholder in the hospital considers infection control their duty. Involving all staff members and creating awareness around the importance of cleaning and disinfection can deliver encouraging results that improve patient safety and reduce costs.