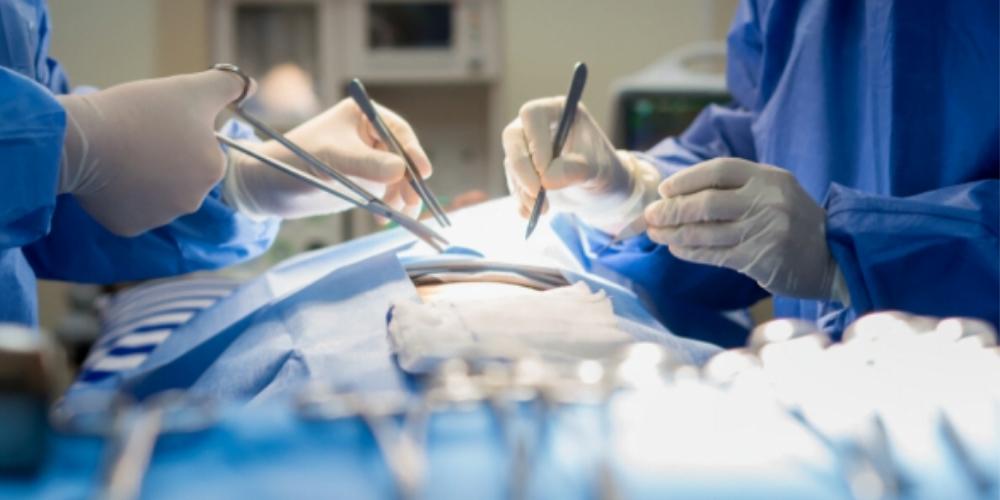
The National Institute for Health Research (NIHR) in the United Kingdom recently funded an international effort led by the University of Birmingham (UK) which set forth nine recommendations that the group believes should be urgently implemented across hospitals world-wide in the fight against surgical site infection (SSI).
Surgical site infections pose significant dangers and delays to the recovery of patients. SSI is the most common complication following abdominal surgery, affecting 9% of patients in high-income countries, such as the United Kingdom and the United States, and 17% of patients in low- and middle-income countries (LMICs). Operating room infections such as these result in patients experiencing pain and discomfort, thus forcing them to extend their recovery time and miss normal day-to-day activities such as work.
On an annual basis, at least 4.2 million people across the world will die within 30 days of surgery due a surgical site infection. This number comprises 7.7% of all deaths globally, which makes it the third greatest contributor, after stroke and ischaemic heart disease.
Moreover, the number of deaths from surgical site infections is more than those from all causes related to HIV, tuberculosis, and malaria combined (2.97 million). It has been estimated that operating room infections and deaths related to them will cost the world economy $12.3 trillion in lost GDP by 2030. Therefore, healthcare organizations must take new measures for infection prevention to increase patient safety and reduce the costs associated with the effects of SSI.
These additional costs associated with surgical site infections can cause financial hardship for patients, especially those who are under-resourced. Operating room infections cause a three-fold increase in the risk of postoperative death. Moreover, clinicians charged with treating surgical site infections face the hurdle of increased antibiotic resistance occuring in patients. Therefore, the emphasis is placed on infection prevention and minimizing the risk of SSI in operating rooms.
The NIHR-funded study was published in the British Journal of Surgery and titled Delphi prioritization and development of global surgery guidelines for the prevention of surgical‐site infection. These new guidelines will support surgeons in practicing key interventions that have been found to reduce the risk of surgical site infections.
The study brought together expert surgeons from fourteen countries across Europe, Africa, Latin America, and South Asia. The group identified nine evidence-based interventions for infection prevention that can be implemented on a global level at a relatively low cost.
Dr. Aneel Bhangu, Consultant Surgeon and Senior Lecturer at the NIHR Global Health Research Unit on Global Surgery at the University of Birmingham commented: “We’ve estimated that around 20 million patients develop surgical site infections worldwide each year following abdominal surgery. . .The Global Surgery Guideline for the Prevention of Surgical Site Infection has identified practical steps that all hospitals should urgently take to both reduce avoidable infections and the spread of antimicrobial resistance.
Based on their research, the group gives the following recommendations for infection prevention:
- Ensuring patients have had a full body wash with clean water and soap before operation.
- Selecting antibiotic prophylaxis according to published antibiotic prescribing guidelines.
- Administering antibiotic prophylaxis to all patients undergoing clean-contaminated, contaminated or dirty surgery.
- Administering antibiotic prophylaxis intravenously within 60 minutes before skin incision.
- Administering a repeat dose of antibiotic prophylaxis if the duration of operation is longer than the half-life of the antibiotic given.
- Not routinely continuing prophylactic antibiotics beyond 24 hours after operation.
- Ensuring scrub teams decontaminate their hands before surgery using antiseptic surgical solution.
- Preparing the skin at the surgical site immediately before incision, using antiseptic preparation
- Providing supplemental oxygen during surgery under general anesthetic
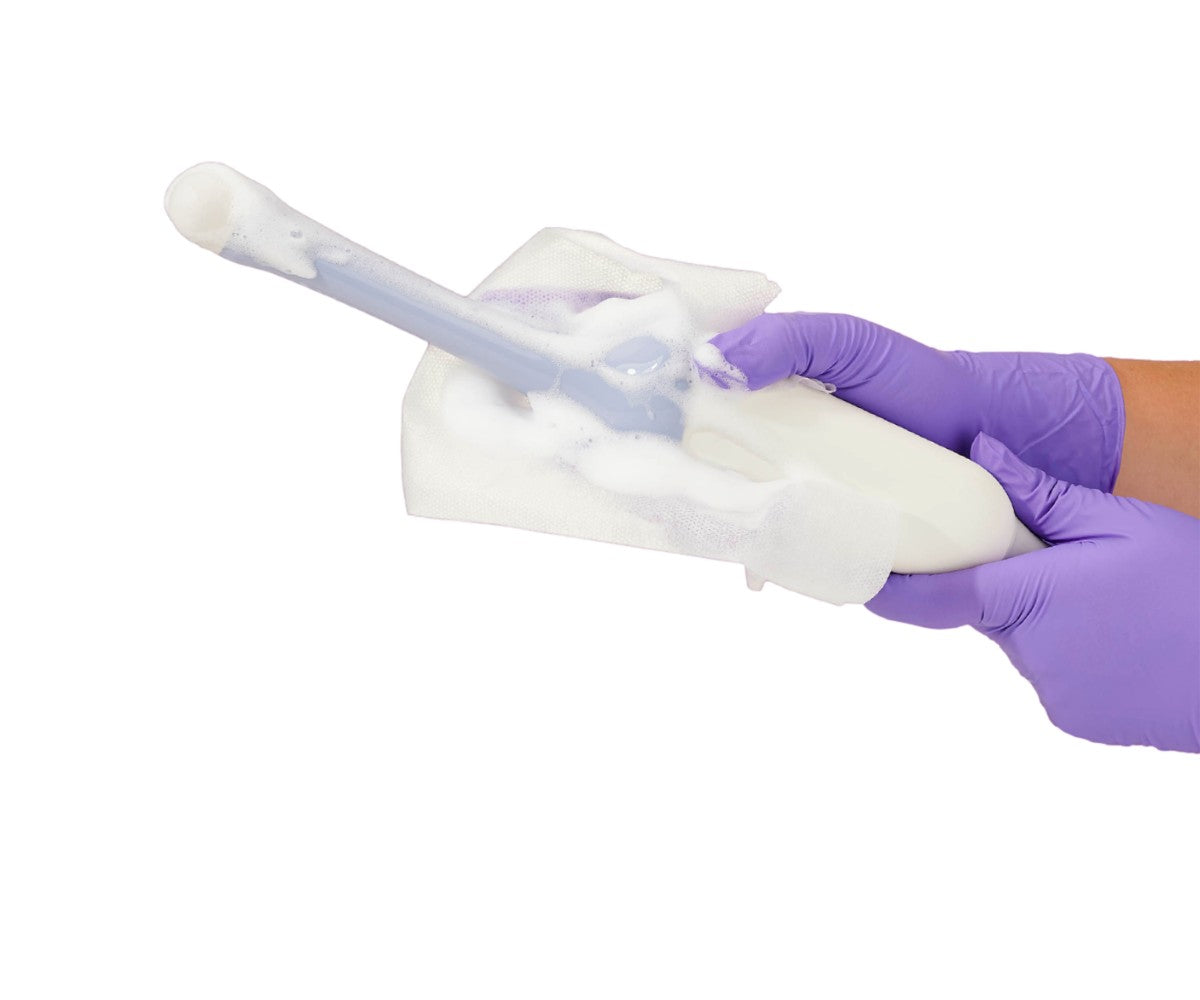
Furthermore, infection preventionists with operating rooms in their purview can promote proper disinfection practices, such as those for medical imaging equipment, to reduce the risk of healthcare-associated infections. Per CDC guidelines, all equipment and devices should be covered with sterile equipment drapes, such as those for c-arms and ultrasound machines, to preserve the sterile field. Imaging equipment, such as ultrasound transducers, can also be covered with sterile probe covers. In addition, surgical staff can disinfect equipment in the OR with hospital-grade disinfectant sprays and wipes to kill surface bacteria. Taking steps such as these reduces the risk of transmission via environmental surfaces, and as a result, improves patient safety.
Please note that the recommendations mentioned in this article are from the NIHR and the University of Birmingham's research group, not EDM Medical Solutions. We recommend that readers consult with their practice's infection control and prevention department before implementing any of the aforementioned recommendations.